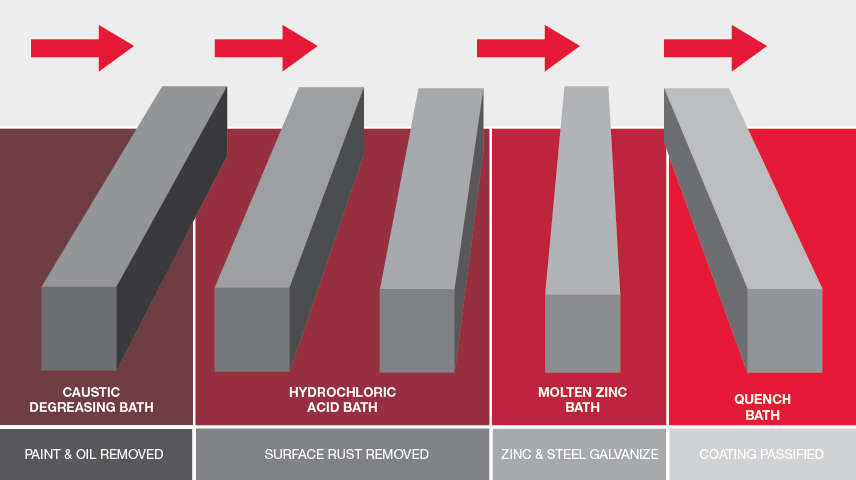
An initial rigorous inspection is conducted to ensure suitability for galvanizing and includes checks for pre-existing coatings and that appropriate holes are in place for ventilation, drainage and hanging. The steel is then cleaned and prepped. Items are suspended on hanging frames and placed in a series of pre-treatment chemical baths to remove rust, contaminants and light pre-existing coatings. Once clean, they are moved into a bath of molten zinc with a nominal operating temperature of 450°C.
The coating is achieved when zinc reacts with iron contained in the steel’s surface. A unique layered protective system is formed as the zinc “galvanizes” with the base metal. Immersion in the bath ensures corners, sealing edges, and all internal and external recesses are coated.
Unlike paint-based systems, the coating metallurgically bonds with the entire surface area of the item and does not shrink from the edges of the steel sections. Three alloying layers, gamma, delta and zeta, form on the surface of the steel. These layers are harder than the base metal, which is typically 150 DPN (diamond pyramid number), and provide the durability and high resistance to abrasion for which hot-dip galvanizing is recognised. Iron content through the layers ranges from 6% to 25% producing hardness levels between 179 DPN and 244 DPN. Eta, a relatively soft, pure zinc fourth layer forms on top. Should this outer layer sustain impact or damage, the harder inner layers continue to offer abrasion resistance together with cathodic protection if the bare metal is exposed.
At the completion of these steps, the item is moved to a quench tank where the steel is cooled to avoid undesirable reactions to the newly coated surface from atmospheric conditions.
Before an item leaves the galvanizing plant, it is thoroughly inspected and fettled to remove any sharp and dangerous edges that may have formed as part of the hot-dip galvanizing process. In addition to the physical barrier the four layers provide, a fifth layer, the patina, will form over a period of time after the item is dispatched. The patina is a series of films on the surface initially consisting of zinc oxide and zinc hydroxide. A final transition occurs as the oxides react to carbon dioxide in the air forming a dull grey zinc carbonate coating. It is the formation of the zinc carbonate film that changes the lustre of previous bright coatings to a matte weathered appearance.
Subject to the conditions of the immediate environment in which the item is located, this transition may occur quickly, or over a period of months. The formation of the patina (weathering) completes the protective armour of the hot-dip galvanized coating and is critical to long-term corrosion protection.